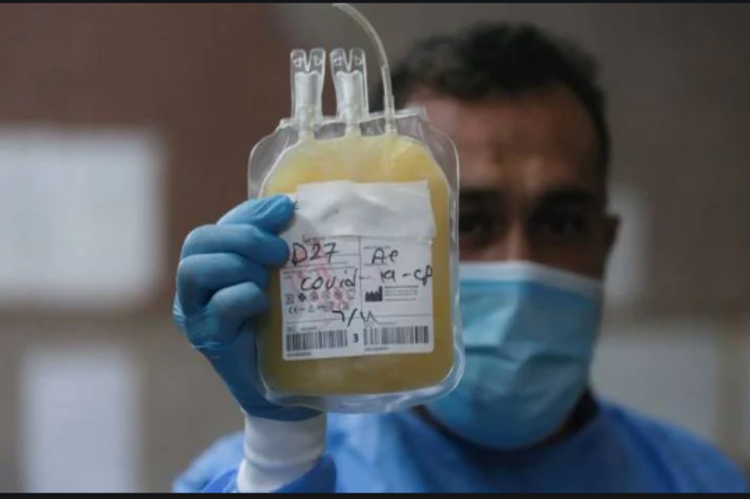
The United States Food and Drug Administration has given emergency authorisation for the use of plasma to treat coronavirus patients.
The technique uses antibody-rich blood plasma from people who have recovered from the disease, and has already been used on over 70,000 people in the US.
Read Also
President Donald Trump said the treatment could reduce deaths by 35%.
It comes a day after he accused the FDA of impeding the rollout of vaccines and therapeutics for political reasons.