Pfizer Inc announced on Thursday that it has signed a $5.29 billion deal with the US government to deliver 10 million courses of its COVID-19 oral antiviral drug starting this year.
The oral drug could be a promising new weapon in the fight against the pandemic, as it can be used as an early at-home treatment to help prevent COVID-19 hospitalizations and deaths.
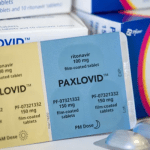
Pfizer filed for US approval of Paxlovid on Tuesday, stating that it expects to manufacture 180,000 treatment courses by the end of next month and at least 50 million courses by the end of 2022.
The company announced earlier this month that the drug reduced the likelihood of hospitalization or death in adults at risk of severe disease by 89 percent.
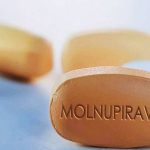
The findings suggest that Pfizer’s drug outperforms Merck & Co Inc’s Molnupiravir, which was shown last month to cut the risk of death or hospitalization in COVID-19 patients at high risk of serious illness in half.
Adults with mild-to-moderate Covid-19 infections who are at risk of becoming seriously ill should take the drug, according to Pfizer. This is similar to how other drugs are used to treat the disease right now.
The US government has also signed a $2.2 billion contract for Merck drug courses.
Pfizer Inc announced on Thursday that it has signed a $5.29 billion deal with the US government to deliver 10 million courses of its COVID-19 oral antiviral drug starting this year.
The oral drug could be a promising new weapon in the fight against the pandemic, as it can be used as an early at-home treatment to help prevent COVID-19 hospitalizations and deaths.
Pfizer filed for US approval of Paxlovid on Tuesday, stating that it expects to manufacture 180,000 treatment courses by the end of next month and at least 50 million courses by the end of 2022.
The company announced earlier this month that the drug reduced the likelihood of hospitalization or death in adults at risk of severe disease by 89 percent.
The findings suggest that Pfizer’s drug outperforms Merck & Co Inc’s Molnupiravir, which was shown last month to cut the risk of death or hospitalization in COVID-19 patients at high risk of serious illness in half.
Adults with mild-to-moderate Covid-19 infections who are at risk of becoming seriously ill should take the drug, according to Pfizer. This is similar to how other drugs are used to treat the disease right now.
The US government has also signed a $2.2 billion contract for Merck drug courses.
Pfizer Inc announced on Thursday that it has signed a $5.29 billion deal with the US government to deliver 10 million courses of its COVID-19 oral antiviral drug starting this year.
The oral drug could be a promising new weapon in the fight against the pandemic, as it can be used as an early at-home treatment to help prevent COVID-19 hospitalizations and deaths.
Pfizer filed for US approval of Paxlovid on Tuesday, stating that it expects to manufacture 180,000 treatment courses by the end of next month and at least 50 million courses by the end of 2022.
The company announced earlier this month that the drug reduced the likelihood of hospitalization or death in adults at risk of severe disease by 89 percent.
The findings suggest that Pfizer’s drug outperforms Merck & Co Inc’s Molnupiravir, which was shown last month to cut the risk of death or hospitalization in COVID-19 patients at high risk of serious illness in half.
Adults with mild-to-moderate Covid-19 infections who are at risk of becoming seriously ill should take the drug, according to Pfizer. This is similar to how other drugs are used to treat the disease right now.
The US government has also signed a $2.2 billion contract for Merck drug courses.
Pfizer Inc announced on Thursday that it has signed a $5.29 billion deal with the US government to deliver 10 million courses of its COVID-19 oral antiviral drug starting this year.
The oral drug could be a promising new weapon in the fight against the pandemic, as it can be used as an early at-home treatment to help prevent COVID-19 hospitalizations and deaths.
Pfizer filed for US approval of Paxlovid on Tuesday, stating that it expects to manufacture 180,000 treatment courses by the end of next month and at least 50 million courses by the end of 2022.
The company announced earlier this month that the drug reduced the likelihood of hospitalization or death in adults at risk of severe disease by 89 percent.
The findings suggest that Pfizer’s drug outperforms Merck & Co Inc’s Molnupiravir, which was shown last month to cut the risk of death or hospitalization in COVID-19 patients at high risk of serious illness in half.
Adults with mild-to-moderate Covid-19 infections who are at risk of becoming seriously ill should take the drug, according to Pfizer. This is similar to how other drugs are used to treat the disease right now.
The US government has also signed a $2.2 billion contract for Merck drug courses.
Pfizer Inc announced on Thursday that it has signed a $5.29 billion deal with the US government to deliver 10 million courses of its COVID-19 oral antiviral drug starting this year.
The oral drug could be a promising new weapon in the fight against the pandemic, as it can be used as an early at-home treatment to help prevent COVID-19 hospitalizations and deaths.
Pfizer filed for US approval of Paxlovid on Tuesday, stating that it expects to manufacture 180,000 treatment courses by the end of next month and at least 50 million courses by the end of 2022.
The company announced earlier this month that the drug reduced the likelihood of hospitalization or death in adults at risk of severe disease by 89 percent.
The findings suggest that Pfizer’s drug outperforms Merck & Co Inc’s Molnupiravir, which was shown last month to cut the risk of death or hospitalization in COVID-19 patients at high risk of serious illness in half.
Adults with mild-to-moderate Covid-19 infections who are at risk of becoming seriously ill should take the drug, according to Pfizer. This is similar to how other drugs are used to treat the disease right now.
The US government has also signed a $2.2 billion contract for Merck drug courses.
Pfizer Inc announced on Thursday that it has signed a $5.29 billion deal with the US government to deliver 10 million courses of its COVID-19 oral antiviral drug starting this year.
The oral drug could be a promising new weapon in the fight against the pandemic, as it can be used as an early at-home treatment to help prevent COVID-19 hospitalizations and deaths.
Pfizer filed for US approval of Paxlovid on Tuesday, stating that it expects to manufacture 180,000 treatment courses by the end of next month and at least 50 million courses by the end of 2022.
The company announced earlier this month that the drug reduced the likelihood of hospitalization or death in adults at risk of severe disease by 89 percent.
The findings suggest that Pfizer’s drug outperforms Merck & Co Inc’s Molnupiravir, which was shown last month to cut the risk of death or hospitalization in COVID-19 patients at high risk of serious illness in half.
Adults with mild-to-moderate Covid-19 infections who are at risk of becoming seriously ill should take the drug, according to Pfizer. This is similar to how other drugs are used to treat the disease right now.
The US government has also signed a $2.2 billion contract for Merck drug courses.
Pfizer Inc announced on Thursday that it has signed a $5.29 billion deal with the US government to deliver 10 million courses of its COVID-19 oral antiviral drug starting this year.
The oral drug could be a promising new weapon in the fight against the pandemic, as it can be used as an early at-home treatment to help prevent COVID-19 hospitalizations and deaths.
Pfizer filed for US approval of Paxlovid on Tuesday, stating that it expects to manufacture 180,000 treatment courses by the end of next month and at least 50 million courses by the end of 2022.
The company announced earlier this month that the drug reduced the likelihood of hospitalization or death in adults at risk of severe disease by 89 percent.
The findings suggest that Pfizer’s drug outperforms Merck & Co Inc’s Molnupiravir, which was shown last month to cut the risk of death or hospitalization in COVID-19 patients at high risk of serious illness in half.
Adults with mild-to-moderate Covid-19 infections who are at risk of becoming seriously ill should take the drug, according to Pfizer. This is similar to how other drugs are used to treat the disease right now.
The US government has also signed a $2.2 billion contract for Merck drug courses.
Pfizer Inc announced on Thursday that it has signed a $5.29 billion deal with the US government to deliver 10 million courses of its COVID-19 oral antiviral drug starting this year.
The oral drug could be a promising new weapon in the fight against the pandemic, as it can be used as an early at-home treatment to help prevent COVID-19 hospitalizations and deaths.
Pfizer filed for US approval of Paxlovid on Tuesday, stating that it expects to manufacture 180,000 treatment courses by the end of next month and at least 50 million courses by the end of 2022.
The company announced earlier this month that the drug reduced the likelihood of hospitalization or death in adults at risk of severe disease by 89 percent.
The findings suggest that Pfizer’s drug outperforms Merck & Co Inc’s Molnupiravir, which was shown last month to cut the risk of death or hospitalization in COVID-19 patients at high risk of serious illness in half.
Adults with mild-to-moderate Covid-19 infections who are at risk of becoming seriously ill should take the drug, according to Pfizer. This is similar to how other drugs are used to treat the disease right now.
The US government has also signed a $2.2 billion contract for Merck drug courses.