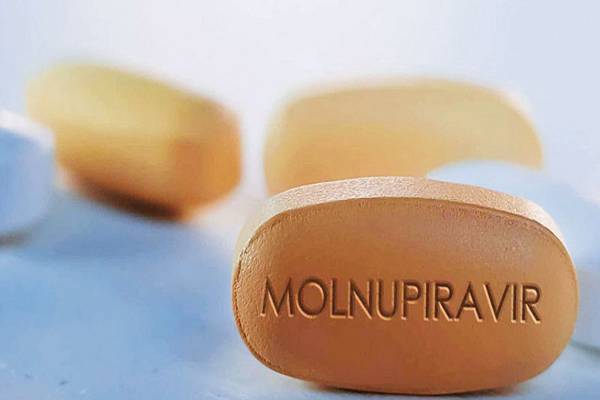
Britain became the first country in the world on Thursday to approve a potentially game-changing COVID-19 antiviral pill developed jointly by Merck and Ridgeback Biotherapeutics in the fight against the pandemic.
According to the UK’s Medicines and Healthcare Products Regulatory Agency, the drug ‘Molnupiravir’ should be used as soon as possible after a positive COVID-19 test and within five days of the onset of symptoms, based on clinical data (MHRA).
This is the first oral antiviral treatment for COVID-19 to be approved, and the approval comes ahead of any potential regulatory approval in the United States. This month, US advisers will vote on whether Molnupiravir should be approved.
Vaccines have been the mainstay of treatment for the pandemic, which has killed more than 5.2 million people worldwide. Other treatments, such as Gilead’s infused antiviral Remdesivir and the generic steroid dexamethasone, are usually given only after a patient has been admitted to the hospital.
Merck’s Molnupiravir has been closely watched since data last month showed it could halve the chances of dying or being hospitalized for those most at risk of developing severe COVID-19 when given early in the illness.
The drug, which will be marketed in the United Kingdom as Lagevrio, is designed to cause errors in the genetic code of the virus that causes COVID-19 and is taken twice a day for five days.
The British government and the country’s National Health Service (NHS) will confirm how the treatment will be deployed to patients in “due course.”
Read Also
The speedy approval in Britain comes as the government struggles to tame soaring infections.
According to the most recent seven-day average, the country has about 40,000 daily cases of COVID-19. Only the United States, which has five times the population, produces more than 74,000 people per day.
Merck said in a separate statement that it expects to manufacture 10 million courses of the treatment by the end of this year, with at least 20 million by 2022.
The U.S. based drug maker’s shares were up 2.1% at $90.54 before the market open.
Pfizer and Roche are also racing to develop easy-to-administer antiviral pills for COVID-19. Pfizer last month began a large study of its oral antiviral drug for the prevention of COVID-19 in people exposed to the coronavirus.
Merck’s Molnupiravir is also being studied in a late-stage trial for preventing infection.